Arkansas’ vaccine distribution plan still in the working stages as COVID-19 cases spike statewide
November 23-29, 2020
Arkansas’ 50-page plan to roll out a COVID-19 vaccine is still in the working stages but represents a major undertaking to fully immunize the state’s more than 3 million residents spread across 75 mostly rural counties.
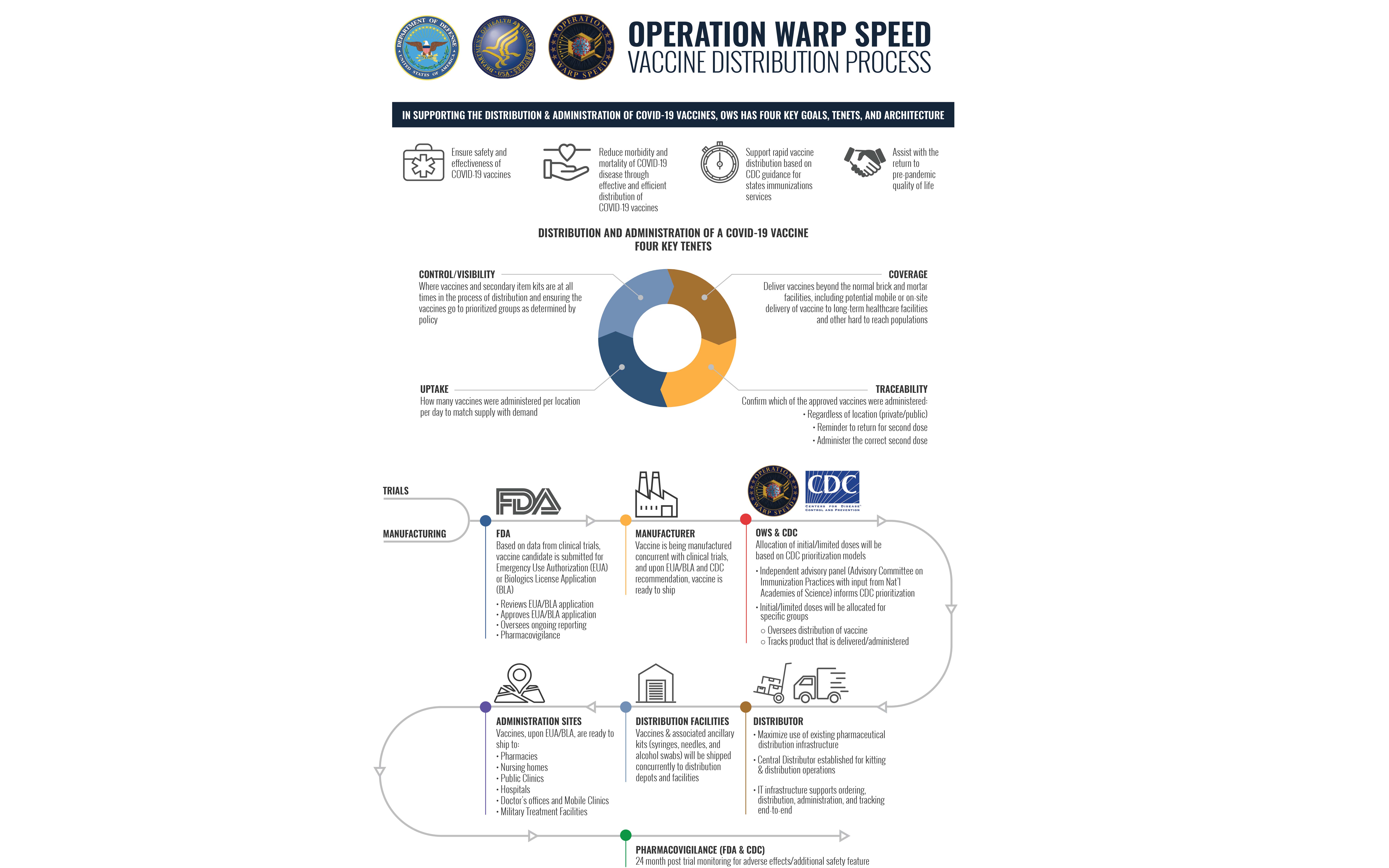
Under the Trump administration’s Operation Warp Speed program to accelerate efforts to find, produce and deliver vaccine doses to the American people by January 2021, all 50 states are required to draft comprehensive plans to distribute the cures develop and research by a host of pharmaceutical firms and manufacturers.
In September, the U.S. Departments of Health and Human Services and Department of Defense released a 11-page strategic plan to distribute a COVID-19 vaccine early to Americans who wanted to receive one, or at least 90% of the estimated 330 million residents counted in the recent 10-year census.
“The leadership of OWS has committed to being transparent with Congress, the media, and the American people. OWS has provided regular briefings on topics of interest to Congress and the media and will continue to provide updates and announcements as OWS reaches new milestones,” HHS and Pentagon officials said. “With support provided through emergency supplemental and flexible discretionary funding, OWS has now made strong progress toward a safe and effective COVID-19 vaccine, with multiple candidates in Phase 3 clinical trials.”
Since Pfizer and Moderna have recently announced successful COVID-19 investigational candidates ahead of schedule after the Nov. 3 election, plans are already underway by the biotech firms to produce hundreds of millions of vaccine doses under a speedy production timetable before the end of 2020. Both pharmaceutical giants said on Nov. 9 and 16 they expect their respective vaccines to receive emergency authorization by the U.S. Food & Drug Administration in a few weeks.
Operation Warp Speed officials, including HHS Secretary Alex Azar and Operation Warp Speed Chief Operating Officer Gen. Gus Parna, have stated publicly in the wake of the Pfizer and Moderna developments that the Trump administration is capable and ready to begin distributing COVID-19 vaccines later this year.
“Ensuring access and affordability of the COVID-19 vaccine for all Americans is a top priority for the Trump Administration. We are leveraging the existing private sector infrastructure to get safe and effective vaccines supported by Operation Warp Speed into communities and into arms as quickly as possible with no out-of-pocket costs,” Azar said on Nov. 13.
“I believe that the mission we have – to develop, manufacture, and deliver safe and effective vaccines – is moving in the right direction. And I am just going to keep my head down and drive to that end,” added Parna, a U.S. Army general who is coordinating the national COVID-19 vaccination program across federal, state, local, tribal, and territorial governments and among many public and private partners.
Under Gov. Hutchinson’s leadership and foresight, Arkansas had already begun pandemic planning in early 2020 before COVID-19 was declared a global health crisis by the World Health Organization on March 11. The Arkansas Health Department’s Emergency Operations Center first began revamping the state’s 12-year hold pandemic flu plan as the first confirmed COVID-19 confirmed alighted in the U.S. in -January.
By early March, state health officials had met several times to update state’s pandemic plan to integrate specific data related to a COVID-19, including improving Arkansas’ personal protection equipment supply requirements and developing a statewide coalition and emergency response to distribute a vaccine to all areas of the state.
According to state Department of Health spokeswoman Danyelle McNeill, Arkansas’ COVID-19 Vaccination Plan template prepared for the CDC is still under review and is a “working document” that has been reviewed and updated three times through Oct. 16.
“It is subject to change,” McNeill told The Daily Record in response to a query concerning a final Arkansas plan. “Yes, we are working closely with the CDC on the plan and we will submit a final version.”
Although not yet final, Arkansas’ COVID-19 distribution strategy will bring to bear a massive integration of local, state and federal government resources, ranging from CDC and HHS back channels to local health units in each of Arkansas 75 counties. In addition, Gov. Hutchinson and ADH officials have brought together an unprecedent public-private partnership that includes the business community, hospitals, health care associations, the nursing home industry, pharmacies and drug stores, homeless shelters, the nonprofit community, and local and county municipal and emergency management offices.
Health Department’s three-phase distribution plan targets healthcare workers, first responders
Under Arkansas namesake COVID-19 Vaccination Program, state health officials would prioritize populations that would receive the vaccine first in three phases. Under a Phase I-A scenario that envisions limited COVID-19 doses, the first group to receive the vaccine would be health care personnel likely to be exposed to patients with COVID-19. That group would include Arkansas residents working in hospitals, home health care, primary care clinics, dialysis treatment centers, long-term care facilities, plasma and blood donation workers, public health nurses, school and university health clinics, and ADH local health units.
Also, health care workers providing testing or vaccinations for COVID-19, first-responders and emergency preparedness workers, and essential government leaders would be administered initial doses of the COVID-19 vaccine in the initial stages.
Phase 1-B of the state’s plan would target people at increased risk for severe illness from COVID-19, including minority populations, those with underlying medical conditions, and people 65 years or older. That plan would also target local daycare workers, employees at correction facilities, K-12 teachers and staff, law enforcement, laborers in the state’s poultry and meatpacking industry, and other government personnel.
In Phase 2, as increased supplies of the vaccine are available, COVID-19 shots would be expanded to all Phase 1 population not previously covered in health care settings such as doctor’s offices, dental clinics, and pharmacies. Next in line would be critical workers in utilities services, transportation, and local grocery stores, followed by workers at food manufacturing plants, nursing homes, and state universities.
As vaccine supplies continued to grow and demand for COVID-19 shots slowed, state health care officials would expand the inoculation network in Phase 3 to target hard-to-reach populations, the homeless and areas with low uptake rates to reach those wary of vaccines or getting shots.
To identity key populations in the state’s phased approach, ADH said it will follow the guidance prepared by the CDC and the National Academies of Sciences, Engineering, and Medicine (NASEM) to identify Arkansas’s critical populations for COVID-19 vaccination.
Also, the Trump administration’s new Operation Warp Speed (OWS) Tiberius platform helps all 50 integrates the related manufacturing, supply chain, allocation, state and territory planning, delivery and administration of both vaccine products and ancillary kits.
Health Department official said they will also continue to work closely with the Arkansas State Data Center at the University of Arkansas at Little Rock (UALR) to update data on Arkansas’ growing population, which topped three million for the first time in 2017.
Other key sections of the state’s COVID-19 vaccination working plan provide details on ordering and distributing and managing vaccine stockpiles, CDC-required documentation, monitoring and reporting, safety protocols, and handling and storage of time-sensitive coronavirus drug supplies. Both vaccine candidates announced by Pfizer and Moderna that are expected to be receive emergency use authorization by the U.S. Food and Drug Administration in the coming weeks must be stored in very cold, secure environments to remain effective, officials said.
What is not known is how Arkansas will pay for costs associated with rolling out the COVID-19 cure-alls in Arkansas in late December or early January. House and Senate leaders are in ongoing talks with the Trump administration to provide billions of dollars in emergency funds COVID-19 relief, but Congress has signaled that state will be responsible to finance their own vaccine distribution plans. President-elect Joe Biden has also proposed a $25 billion testing and distribution plan once he takes office in early 2021.
And according to recent filings with the federal Securities and Exchange Commission, Moderna revealed that its mRNA-based COVID-19 vaccine candidate, mRNA-1273, will cost between $32 to $37 per dose for some customers.” In late July, Moderna said it had already received $400 million of customer deposits to deliver mRNA-1273 once it received FDA approval.
Also in July, Pfizer and its German vaccine partner, BioNTech, entered a $1.95 billion contract with the Trump administration to provide 100 million doses of their COVID-19 vaccine, BNT162b2, by the end of this year, putting vaccine costs at $19.50 per dose. Other COVID-19 investigational vaccines in the U.S. pipeline range from $10 to $50 per dose.
In response to a query from The Daily Record, Katie Beck, spokeswoman for Gov. Hutchinson’s office, did not provide any details concerning potential costs associated with Arkansas’ distribution of a COVID-19 vaccine. Under the Coronavirus Aid, Relief and Economic Security (CARES) Act approved by Congress in late March, Gov. Hutchinson created a 15-person task force to make recommendations for $1.25 billion in coronavirus relief funding that Arkansas must spend by Dec. 30.
On Nov. 10, the Arkansas CARES Act Steering Committee said the bicameral Legislative Council has depleted all but $70 million CARES Act funds that must be spent on coronavirus relief and cannot be comingled with other state revenues. The state, however, has put $450 million in CARES Act funding on hold that must be allocated and distributed by year’s end.
DFA Secretary Larry Walther, Arkansas chief budget officer, said department officials are assessing how this money will be used ahead of the Dec. 30 deadline. Some states like Illinois and New York are requesting proposals from the private sector to help in the distribution of a COVID-19 vaccine.
DFA spokesman Scott Hardin told the Daily Record that there is currently $18.9 million in unallocated CARES Act funds and $407 million that is allocated but has not been distributed. “As you know the deadline to spend this funding is the end of December,” said Hardin. “We are in communication with our congressional delegation regarding the potential for a federal extension of the deadline.”
Regarding vaccine distribution costs, Hardin added there may be some non-CARES Act funds in place that the Health Department may access to support the state’s vaccination program. He also noted that the CARES Act Steering Committee, which includes Walther and other DFA executive staff, has discussed the possibility of funding supporting vaccine distribution.
“However, it has not been formally considered or presented to the committee,” Hardin said Nov. 17.
A day later, at the state CARES Act Steering Committee meeting that was held virtually on Wednesday (Nov. 18), state Health Department officials told the panel that funding will not be needed for vaccine distribution as costs will be covered in full by Operation Warp Speed. Yet, the price tag for that plan in Arkansas and the other 49 states is still unknown.
PHOTO CAPTION: (Photo by National Institutes of Health)
From left: Drs. Anthony Fauci, head of the National Institutes of Health’s National Institute of Allergy and Infectious Diseases; Matt Hepburn of the Defense Department; and Francis Collins, NIH director, stand for a photo at NIH in Bethesda, Md., July 27, 2020, while launching the first efficacy trial of an investigational vaccine for COVID-19 under Operation Warp Speed.