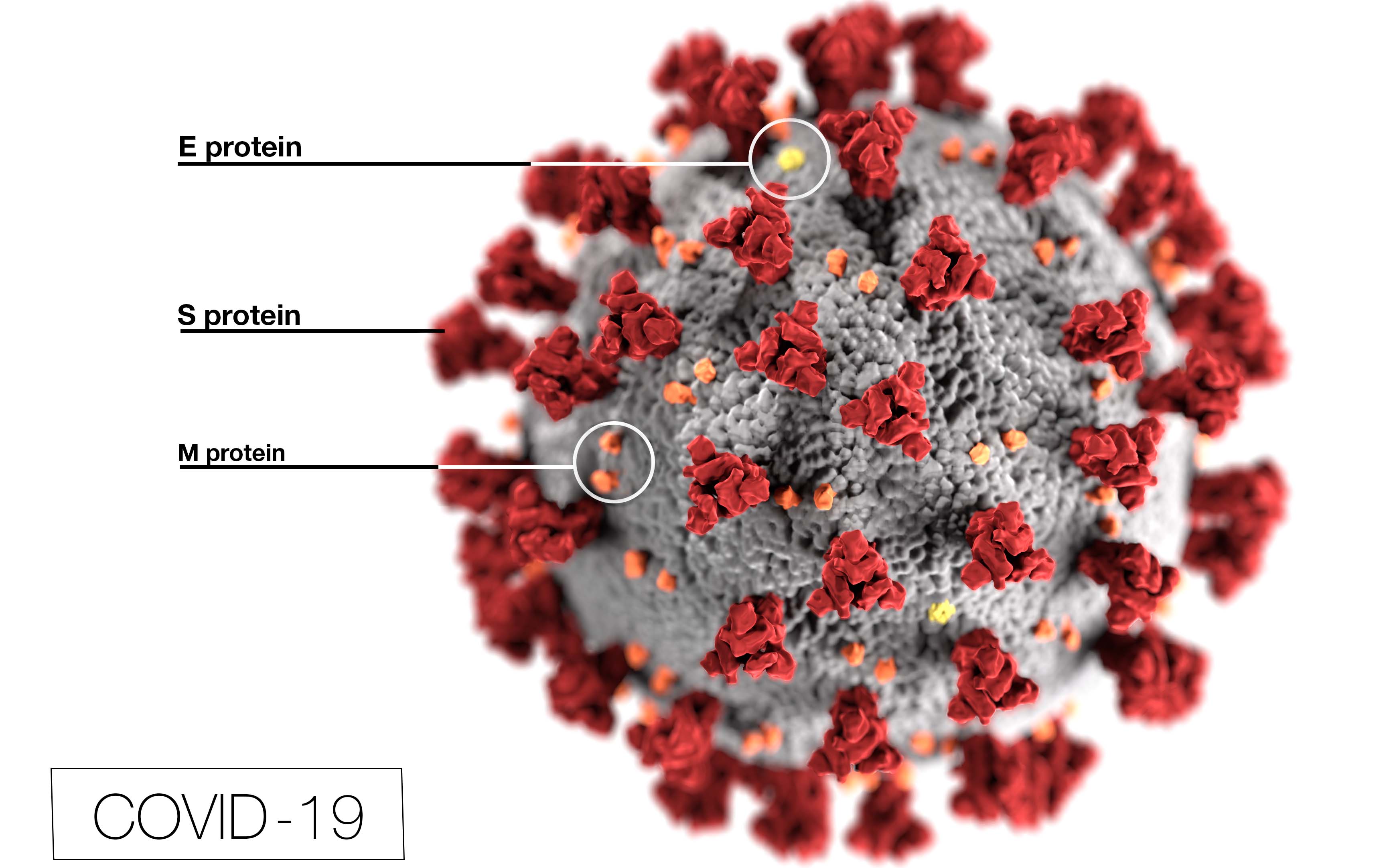
COVID-19 Small Business Resources
November 23-29, 2020
Gov. Asa Hutchinson creates COVID-19 Winter Task Force to mitigate spike in coronavirus cases, hospitalizations
Gov. Asa Hutchinson on Nov. 13 issued an executive order creating the COVID-19 Winter Task Force as the number of coronavirus cases in Arkansas continue to spike to record levels across the state. The new task force includes 19 physicians, state officials, and health care executives to advise Hutchinson as the state combats the likely additional challenges of the pandemic this winter.
In its Nov. 13 COVID-19 update, the Arkansas Department of Health reported a record number of new COVID-19 cases and hospitalizations across the state at 2,312 and 826, respectively. On Nov. 16, Health Department officials reported 1,308 new cases, 16,485 active cases and 861 hospitalized, which is up 31 from Sunday and a new record.
Of those hospitalized, there were 123 on ventilators and 42 deaths added to the state’s total of 2,225. The Health Department reported that the top counties for new cases are Washington, 161; Pulaski, 130; Benton, 70; Sebastian, 64; and Craighead, 63.
“The new cases are higher than last Monday, and this may be an indication we are in for a tough week ahead. The 42 new deaths is regrettably an all-time high in a single day,” said Hutchinson. “While there is good news on the vaccine front this morning, we have to work together to reduce cases, hospitalizations and save lives.”
Concerning the newly created task force, Gov. Hutchinson will serve as chair, and Dr. Greg Bledsoe, Arkansas Surgeon General, will serve as vice chair. Additional members could be added to the citizens’ task force as the governor deems necessary.
Other members of the 19-person panel include:
• Larry Shackelford, President and CEO of Washington Regional Medical Center;
• Chris Barber, President and CEO of St. Bernard’s Healthcare;
• Scott Street, CEO of Medical Center of South Arkansas;
• Dr. Cam Patterson, Chancellor of the University of Arkansas for Medical Sciences;
• Rachel Bunch, Executive Director of Arkansas Health Care Association;
• Bo Ryall, President and CEO of Arkansas Hospital Association;
• Troy Wells, President and CEO of Baptist Health;
• Ryan Gehrig, President of Mercy Hospital, Fort Smith;
• Major General Kendall Penn, Adjutant General, Arkansas National Guard;
• A.J. Gary, Director of Arkansas Department of Emergency Management;
• Dr. José Romero, Secretary of Arkansas Department of Health;
• Dr. Jerrilyn Jones, Arkansas Department of Health;
• Dr. Jennifer Dillaha, Arkansas Department of Health;
• Dr. Naveen Patil, Arkansas Department of Health;
• Dr. Keyur Vyas, University of Arkansas for Medical Sciences;
• Phillip Gilmore, CEO of Ashley County Medical Center;
• Dr. Steven Collier, CEO of ARcare;
• Ron Peterson, President and CEO of Baxter County Regional Medical Center.
Emergency Physicians urge Thanksgiving Day precautions to avoid “COVID-19 Super Spreader” event
The American College of Emergency Physicians (ACEP) recommends that holiday hosts and guests heed the Centers for Disease Control and Prevention (CDC)’s holiday safety recommendations this year by prioritizing efforts to prevent the spread of COVID-19 and take steps to protect the health and safety of friends and family.
“If you are planning to get together on Thanksgiving, it is a good idea to reduce the risks that invite COVID-19 into your home,” said Dr, Mark Rosenberg, president of the American College of Emergency Physicians (ACEP). “Even a small gathering of family or close friends can still contribute to the spread of the virus.”
Emergency physicians makes these specific recommendations:
• Remember anyone can get or spread COVID-19. Close friends and family with whom you don’t live with can still contract and spread the virus to you the same way a stranger could.
• Trim the guest list. Rather than a specified “safe” number of guests, public health experts suggest that hosts determine the size of a gathering by how many guests from different households can remain at least six feet apart. Note that a “household” is made of people who live in the same house every day. Family members who are close but don’t live at home, such as college students visiting for the holiday, are considered a separate household in public health terms.
• Cover your face and maintain your distance. It may be difficult but try to avoid hugs and handshakes. People should also cover their face when they are not eating or drinking.
• Stay outside and stay safer. If it is possible, hosting a small event outside instead of inside is preferable.
• Encourage good hygiene. Hosts should make sure that bathrooms have plenty of soap so guests can frequently wash their hands and single-use towels.
Even with these recommendations during the holiday season, the safest option for some will be to stay home. Do not attend an in-person gathering if you or anyone in your household has been diagnosed with COVID-19 and has not met the CDC’s criteria for when it is safe to be around others, ACEP officials said. Stay home if you show symptoms, if you are waiting for COVID-19 test results, or if you have been exposed to somebody with COVID-19 in the last 14 days.
“Unfortunately, the safest option for older individuals or people with weakened immune systems is to skip in-person gatherings this year,” said Dr. Rosenberg. “It may be disappointing to adjust traditions or modify plans in the short-term, but these decisions can save lives.”
Remember, emergency physicians work 24/7, even on holidays. Do not ignore your symptoms if you think you are having a medical emergency–if something is wrong call 911 or visit your closest emergency department. Emergency departments across the country are taking extensive precautions to adapt and protect patients. If holiday plans go awry, emergency departments are safe and ready for anything or anyone that comes their way.
Federal housing officials extend COVID-19 related loan flexibilities through end of 2020
The Federal Housing Finance Agency (FHFA) announced on Nov. 13 that federal mortgage servicers Fannie Mae and Freddie Mac will extend several loan origination flexibilities that were set to expire at the end of November until Dec. 31, 2020. The changes are to ensure continued support for borrowers during the COVID-19 national emergency, FHFA officials
The FHFA’s extended flexibilities related to the COVID-19 pandemic include alternative appraisals on purchase and rate term refinance loans; alternative methods for documenting income and verifying employment before loan closing; and expanding the use of power of attorney to assist with loan closings.
The FHFA regulates Fannie Mae, Freddie Mac and the 11 federal Home Loan Banks, including the Federal Home Loan Bank of Dallas that supports housing and community development by providing competitively priced advances and other credit products for nearly 815 members and associated institutions in Arkansas, Louisiana, Mississippi, New Mexico and Texas.
Fannie Mae and Freddie Mac provide more than $6.6 trillion in funding for the U.S. mortgage markets and financial institutions. Formally known as the Federal Home Loan Mortgage Corporation (FHLMC), Freddie Mac was created by the federal government in 1970 to expand the secondary market for mortgages that was monopolized by its bigger sibling, Fannie Mae, or the Federal National Mortgage Association.
NIH: Hydroxychloroquine does not benefit adults hospitalized with COVID-19
A National Institutes of Health clinical trial evaluating the safety and effectiveness of hydroxychloroquine for the treatment of adults with coronavirus disease 2019 (COVID-19) has formally concluded that the drug provides no clinical benefit to hospitalized patients.
Though found not to cause harm, early findings in June when the trial was stopped indicated that the drug was not improving outcomes in COVID-19 patients. Final data and analyses of the trial, which was funded by the National Heart, Lung, and Blood Institute (NHLBI), part of NIH, appeared online Nov. 9 in the Journal of the American Medical Association.
The trial, called Outcomes Related to COVID-19 treated with Hydroxychloroquine among Inpatients with symptomatic Disease (ORCHID), began after lab studies and preliminary reports suggested that hydroxychloroquine – commonly used to treat malaria and rheumatic conditions like arthritis – might have promise in treating SARS-CoV-2, the virus that causes COVID-19.
The Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network of NHLBI started the trial in April at 34 hospitals across the United States and enrolled 479 of the expected 510 patients. By June, preliminary evidence indicated hydroxychloroquine was unlikely to offer any benefit. NIH officials said the careful design, implementation, and oversight of the study was key to its results, as well as the recommendation by a data and safety monitoring board (DSMB) to stop the trial early.
“Having a rigorously designed clinical trial that captured patient-centered, clinically meaningful outcomes was critical to reaching the unequivocal conclusions about the use of hydroxychloroquine in COVID-19. ORCHID shows that hydroxychloroquine does not improve clinical outcomes in hospitalized COVID-19 patients,” said James Kiley, Ph.D., director of Division of Lung Diseases at NHLBI. “We hope this clear result will help practitioners make informed treatment decisions and researchers continue their efforts pursuing other possible safe and effective treatments for patients suffering with this disease.”
The ORCHID trial enrolled participants between April 2 and June 19 who were a median age of 57. They included 290 Hispanic and Black participants and 212 female participants. All participants received clinical care as indicated for their condition. Participants were randomly assigned to a treatment group and received 10 doses of either hydroxychloroquine or a placebo over five days.
Researchers then assessed each patient’s clinical status 14 days after being assigned to a treatment group. They used a seven-category scale ranging from one death to seven discharged from the hospital and able to perform normal activities. Researchers also measured 12 additional outcomes, including death that occurred 28 days after the participants’ assignment to a treatment group.
At day 14, those who received hydroxychloroquine and those who received a placebo had a similar health status, with most participants in both groups discharged from the hospital and able to perform a range of activities. The number of participants in both treatment groups who died at day 14 was also similar. At day 28, 25 of 241 patients in the hydroxychloroquine group and 25 of 236 patients in the placebo group had died.
“The finding that hydroxychloroquine is not effective for the treatment of COVID-19 was consistent across patient subgroups and for all evaluated outcomes, including clinical status, mortality, organ failures, duration of oxygen use, and hospital length of stay,” said Dr. Wesley Self, emergency medicine physician at Vanderbilt University Medical Center and PETAL Clinical Trials Network investigator who led the ORCHID trial. He also noted that the finding was consistent with similar trials in the United Kingdom and Brazil.
As of Nov. 2, the CDC has reported more than 9.1 million cases of COVID-19 and more than 230,000 deaths in the U.S. Many other randomized clinical trials are currently evaluating the effectiveness and safety of other agents versus a placebo in the urgent race for effective therapies to treat COVID-19.