First COVID-19 vaccine hits Arkansas, distributed in small doses across U.S.
December 21-27, 2020
Only two days after the federal Food and Drug Administration issued an emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine, the first shipments of the drug began arriving in Arkansas and other states across the U.S. on Monday (Dec. 14) as the total number of deaths topped 300,000 nationwide.
In Arkansas, where coronavirus deaths jumped by 45 to 2,990 just 10 days before Christmas, state Department of Health officials held a media event as several ADH front-line workers began receiving the first COVID-19 vaccinations in Arkansas. Gov. Asa Hutchinson expressed hope on the arrival of Pfizer vaccine’s arrival to the Natural State, but also continues to urge caution as total COVID-19 cases surged to 187,057.
“Today is a hopeful day. After months of work, a COVID-19 vaccine is in Arkansas, and the first vaccine was given to Sherian Kwanisai today,” said Hutchinson. “The FDA should approve additional vaccines soon, and we will be set to cover our long-term care facilities. Brighter days are ahead, but we must continue to follow public health guidelines. This virus continues to rapidly spread, and it’s up to each of us to do our part to slow the spread.”
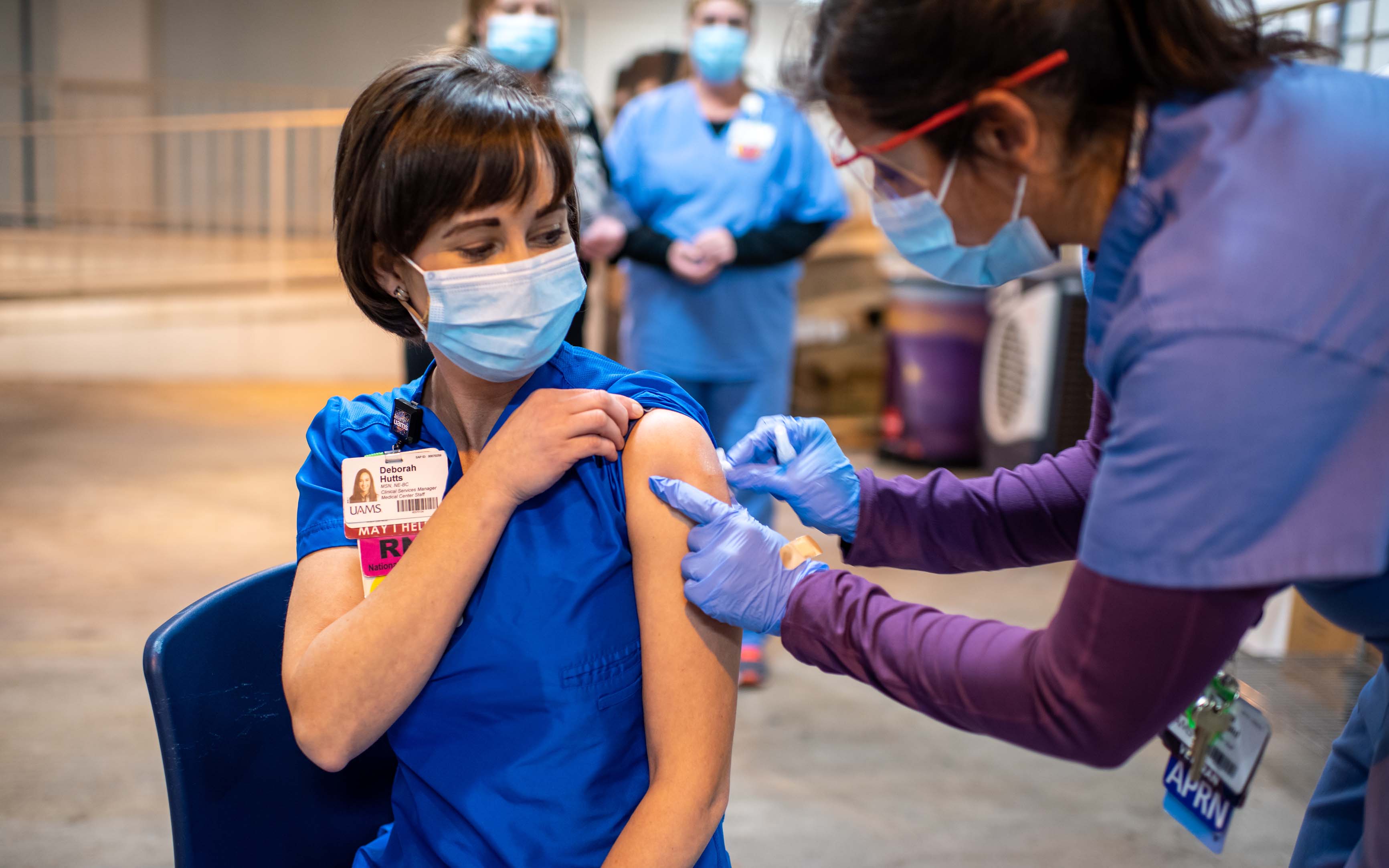
In Central Arkansas, the University of Arkansas for Medical Sciences (UAMS) received its first allocation of 2,000 COVID-19 vaccinations from the Arkansas Department of Health on Dec. 15 and began administering the first vaccines to critical health care employees.
UAMS Chancellor Dr. Cam Patterson said the state’s only health sciences university prioritized employees based on potential exposure and risk of transmitting the virus, with employees working in intensive care units, the Emergency Department, COVID testing sites and units with COVID-positive patients being given the first opportunity to receive the vaccine. This includes physicians, nurses, therapists and other health care workers along with transport, registration, environment services and nutrition services employees. Other health care workers and students who work directly with patients will be offered the vaccine as additional quantities become available.
“We have been anxiously awaiting an effective vaccine for many months,” said Patterson. “We are pleased to be able to offer this protection from the COVID-19 virus to the employees who have put their health and that of their families at risk to care for our patients. They have been under tremendous physical and emotional strain for more than eight months, and knowing they have additional protection from infection will provide a tremendous sense of relief.
“This vaccine is only the first step in overcoming this virus,” he said. “We will continue to require masks, eye protection and other protective gear for the foreseeable future, as we do not yet know how long the vaccine is effective or whether vaccinated people can transmit the virus. We are confident that the vaccine is safe, and we look forward to being able to offer the vaccine to all our employees, students and patients.”
Arkansas has a plan for vaccine distribution that mirrors the national Centers for Disease Control and Prevention (CDC) plan, which places priority on those most impacted by the coronavirus as first in line to receive the vaccine. This includes health care workers and others at higher risk of significant exposure to the virus.
Under Arkansas’ three-phase, COVID-19 Vaccination Program, state health officials are prioritizing populations that receive the vaccine first. Under a Phase I-A scenario that envisions limited COVID-19 doses, the first group to receive would be physicians, nurses and any staff member working in direct contact with COVID-19 patients will also soon receive the first of the two-dose regimen.
According to Gov. Hutchinson, there will be limited supplies of the vaccine before state health officials began distributing the COVID-19 treatment to the general public. As vaccine supplies continued to grow and demand for COVID-19 shots slows, state health care officials would expand the vaccination network in Phase 2 and 3 to a wider net of Arkansas’ 3 million-plus population.
“I received the vaccine today, and I encourage everyone to be vaccinated once it is available to them,” said Dr. Robert Hopkins, a UAMS professor and division director of General Internal Medicine and chair of the National Vaccine Advisory Committee of the U.S. Department of Health & Human Services.
Nationally, Operation Warp Speed COO Gustave Perna said vaccines began moving from Pfizer’s manufacturing facility to the United Parcel Service and FedEx hubs over the past week, and then will go out to the 636 locations nationwide. Those sites were previously identified by U.S. states and territories.
We expect 145 sites across all the states to receive the vaccine on Monday, another 425 sites on Tuesday and the final 66 sites on Wednesday, which will complete the initial delivery of the Pfizer orders for the vaccine,” said Perna, a four-state U.S. Army general who is overseeing the nationwide distribution plan for the Trump administration’s Operation Warp Speed. That nationwide project includes several U.S. government components and public partnerships to move the development, manufacturing and distribution of COVID-19 vaccines, therapeutics and diagnostics.
Nationally, Northwell Health in New Hyde Park, N.Y., made history on Dec. 14 by vaccinating the first person in the U.S. against COVID-19, when it injected Pfizer Inc.’s medication into Sandra Lindsay, an intensive care nurse at Long Island Jewish Medical Center. Ms. Lindsay’s participation kick-started a long-anticipated vaccination deployment program throughout the US, as well as the first phase of Northwell’s three-stage rollout to essential frontline hospital personnel.
“Today is V-Day in our fight against COVID-19,” said Michael Dowling, Northwell president and CEO of the largest health care provider and private employer in New York state. “This truly is a historic day for science and humanity, one in which we here in New York and across the United States have been waiting for quite some time.”
Like most hospitals, Northwell received a limited supply of a few thousand doses to be spread among eight hospitals. The Pfizer vaccine, which will require two injections 21 days apart, has demonstrated 95% efficacy against infection with minimal side effects, and works on messenger RNA (mRNA) technology, which has been in development for several years. mRNA instructs cells in the body to make different proteins.
To vaccinate team members, Northwell said it prepared a three-phase prioritization matrix to help deploy the vaccine to its over 74,000 employees. The plan factors in a person’s work/geographic area, department specialty, job function and age. To prepare for the Pfizer vaccine that needs to extensive cold storage, Northwell invested in procuring more than 20 -70F freezers, which can store about 250,000 doses each. Northwell said also stocked up on extra needles, gloves, swabs to help distribute the vaccine once ready, and the health system has been working together with state and federal officials for a rollout.
Closer to home, Gov. Hutchinson has stated publicly that Arkansas would only receive about 25,000 doses in the first round of distributions. If other vaccines are approved as expected in the coming weeks, that distribution timetable could be accelerated, he said.
The FDA’s Vaccines and Related Biological Products Advisory Committee is meeting on Thursday, Dec. 17, in open session to discuss Emergency Use Authorization (EUA) of the Moderna Inc’s COVID-19 vaccine. Based on the interim safety and efficacy data from Phase 3 clinical trials that slow 95 effectively against COVID-19 will little side effects, Boston-based Moderna has submitted applications for authorizations to several global regulatory agencies, including the FDA.
Moderna is working with the CDC, OWS partners and McKesson, a COVID-19 vaccine distributor contracted by the U.S. government, as well as global stakeholders to be prepared for distribution of its mRNA-1273. If approved by the FDA this week, the East Coast pharmaceutical expects to have approximately 20 million doses of mRNA-1273 ready to ship in the U.S. and remains on track to manufacture 500 million to 1 billion doses globally in 2021.
PHOTO CAPTIONS:
1. Barbara McDonald, an advanced practice registered nurse at UAMS, gets her COVID-19 shot after the first doses of the Pfizer vaccine arrived in Arkansas on Dec. 15. (Photo courtesy of UAMS)
2. From left: Northwell Health President and CEO Michael Dowling, Sandra Lindsay, RN; and Michelle Chester, DNP, at Long Island Jewish Medical Center. (Photo courtesy of Northwell Health)
3. Dr. Moncef Slaoui, left, chief advisor to Operation Warp Speed, and Army Gen. Gustave F. Perna, chief operating officer of OWS, visit a UPS freezer farm in Louisville, Ky. (Photo courtesy of Department of Defense)
4. Deborah Hutts, clinical service manager in the UAMS COVID-19 triage unit, was one of the first Arkansans to receive coronavirus vaccine in Arkansas. (Photo courtesy of UAMS)